同济大学:《大学物理》课程教学资源(电子教案,英文版)第十章 气体动力论

The Kinetic Theory of Gas
The Kinetic Theory of Gas

Agenda Today ·Ideal gas Pressure and temperature micro interpretation) Internal energy of idea gas Distribution law of molecular speed for ideal gas mean free path
Agenda Today • Ideal gas • Pressure and temperature ( micro interpretation) • Internal energy of idea gas • Distribution law of molecular speed for ideal gas • mean free path

The ideal--gas law(理想气体状态方程) m PV=RT M M:molar mass(摩尔质量) R:universal gas constant(普适气体常数) R=8.31J/mol'k
The ideal-gas law(理想气体状态方程) RT M m PV M: molar mass(摩尔质量) R: universal gas constant(普适气体常数) R=8.31J/molk
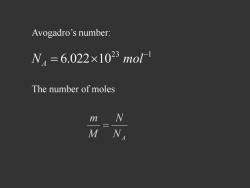
Avogadro's number: N4=6.022×1023mo1 The number of moles N M NA
23 1 6.022 10 N mol A Avogadro’s number: The number of moles

Other forms of idea-gas Law PV-NKT K:Boltzmann's constant 13813/K M 0= RT
PV NkT K : Boltzmann’s constant Other forms of idea-gas Law
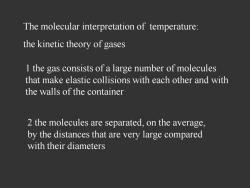
The molecular interpretation of temperature: the kinetic theory of gases 1 the gas consists of a large number of molecules that make elastic collisions with each other and with the walls of the container 2 the molecules are separated,on the average, by the distances that are very large compared with their diameters
The molecular interpretation of temperature: the kinetic theory of gases 1 the gas consists of a large number of molecules that make elastic collisions with each other and with the walls of the container 2 the molecules are separated, on the average, by the distances that are very large compared with their diameters

3 the molecules did not exert force on each other except when they collide 4 there is no preferred position for a molecule and no preferred direction for velocity,when there is no external force
3 the molecules did not exert force on each other except when they collide 4 there is no preferred position for a molecule and no preferred direction for velocity, when there is no external force
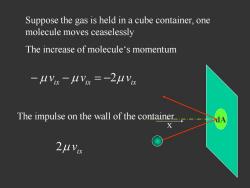
Suppose the gas is held in a cube container,one molecule moves ceaselessly The increase of molecule's momentum -MVis -MlVi =-2MVa The impulse on the wall of the container
x dA The increase of molecule‘s momentum i x i x i x v v 2 v The impulse on the wall of the container ix 2 v Suppose the gas is held in a cube container, one molecule moves ceaselessly

The number of collision the molecule has with the wall in dt The impulse of all the molecule: r dt d force dF dt
dA The number of collision the molecule has with the wall in dt v dt l ix x 2 1 dt l v dI x i x 2 force dt dI dF x ix l v 2 The impulse of all the molecule:

pressure: P=9 since ==- -nn
pressure: x i x Al v A dF P 2 V Nvix 2 since 2 2 2 2 3 1 v v v v x y z 2 2 3 1 P nv nv x
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十一章 热力学(上).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第六章 刚体的转动(下)..pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第六章 刚体的转动(上)..pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第八章 振动.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第九章 机械波(中).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第九章 机械波(下).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第九章 机械波(上).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第七章 引力.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第二章 质点的运动.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第三章 牛顿定律.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第一章 测量(负责人:王治国).pdf
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-13 超导电性.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-12 半导体.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-11 激光.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-08 量子力学简介.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-07 不确定关系.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-06 德布罗意波、实物粒子的二象性.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-05 夫兰克-赫芝实验.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-04 玻尔理论.ppt
- 高等教育出版社:《物理学教程》教材电子教案(PPT课件,马文蔚第四版)第十九章 量子物理 19-03 康普顿效应.ppt
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)原子物理1.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)原子物理2.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第二十一章 相对论.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第二十三章 固体物理.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第二十章 光学(下).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第二十章 光学(中).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十一章 热力学(下).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十七章 交流电路.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十三章 高斯定律.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十九章 电磁波.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十二章 电场与电势(上).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十二章 电场与电势(下).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十五章 磁场(上).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十五章 磁场(下).pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十八章 物质的磁性.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十六章 电磁感应.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)第十四章 直流电路.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)量子1.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)量子2.pdf
- 同济大学:《大学物理》课程教学资源(电子教案,英文版)量子3.pdf
