北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 5 Chemical Equilibrium

Chemical equilibrium PDF文件使用"pdfFactory Pro”试用版本创建w,fineprint.com,c四
Chemical equilibrium PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì

Introduction Chemical reaction moved towards a dynamic equilibrium in which both reactants and products are present but have no further tendency to undergo net change.In some cases,the concentration of products in the equilibrium mixture is so much greater than the concentration of unchanged reactants that for all practical purposes the reaction is 'complete'. However,in many important cases the equilibrium mixture has significant concentration of both reactants and products.In this chapter we see how to use thermodynamics to predict the equilibrium composition under any reaction conditions. PDF文件使用"pdfFactory Pro”试用版本创建w.fineprint.com.cn
Introduction Chemical reaction moved towards a dynamic equilibrium in which both reactants and products are present but have no further tendency to undergo net change. In some cases, the concentration of products in the equilibrium mixture is so much greater than the concentration of unchanged reactants that for all practical purposes the reaction is ‘complete’. However, in many important cases the equilibrium mixture has significant concentration of both reactants and products. In this chapter we see how to use thermodynamics to predict the equilibrium composition under any reaction conditions. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì

Spontaneous chemical reactions We have seen that the direction of spontaneous change at constant temperature and pressure is towards lower values of the Gibbs energy G.The idea is entirely general, and in this chapter we apply it to the discussion of reaction. PDF文件使用"pdfFactory Pro”试用版本创建w,fineprint.com,cn
Spontaneous chemical reactions We have seen that the direction of spontaneous change at constant temperature and pressure is towards lower values of the Gibbs energy ,G. The idea is entirely general, and in this chapter we apply it to the discussion of reaction. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì

.The reaction Gibbs energy Consider the reaction:0=EveB At constant T and P,suppose the reaction advances by d,the corresponding change in Gibbs energy: dGT.p=MAdna+ugdng+ucdnc+.....=>uedng =LA VAdg+uB ved+uc vcd+......=>vB uBds dGT,p=∑%ed5 .(OGla)1p=ΣB4B=△,Gm The reaction Gibbs energy,A,Gm is defined as the slope of the graph of the Gibbs energy plotted against the extent of reaction. PDF文件使用"pdfFactory Pro”试用版本创建耀m,fineprint.com,cn
Consider the reaction: 0=ånBB At constant T and P,suppose the reaction advances by dx,the corresponding change in Gibbs energy: dGT,P=mAdnA+mBdnB+mCdnC+......=åmBdnB =mAnAdx+mBnBdx+mCnCdx+......=ånB mBdx dGT,P=ånB mBdx \(¶G/¶x)T,P=ånB mB=DrGm •The reaction Gibbs energy The reaction Gibbs energy, DrGm, is defined as the slope of the graph of the Gibbs energy plotted against the extent of reaction. PDF 文件使用 "pdfFactory Pro" 试用版本创建 耀www.fineprint.com.cn
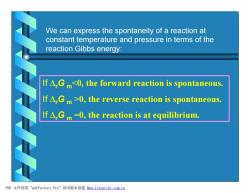
We can express the spontaneity of a reaction at constant temperature and pressure in terms of the reaction Gibbs energy: If A.Gm0,the reverse reaction is spontaneous. If△,Gm-0,the reaction is at equilibrium. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
We can express the spontaneity of a reaction at constant temperature and pressure in terms of the reaction Gibbs energy: If DrG m0, the reverse reaction is spontaneous. If DrG m =0, the reaction is at equilibrium. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn
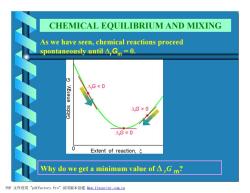
CHEMICAL EQUILIBRIUM AND MIXING As we have seen,chemical reactions proceed spontaneously until A,Gm =0. △,G0 △.G=0 0 Extent of reaction, Why do we get a minimum value of△,Gm? PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint..com,cn
As we have seen, chemical reactions proceed spontaneously until DrGm = 0. Why do we get a minimum value of D rG m? CHEMICAL EQUILIBRIUM AND MIXING PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn
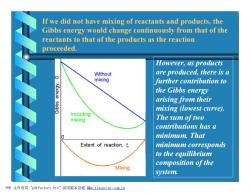
If we did not have mixing of reactants and products,the Gibbs energy would change continuously from that of the reactants to that of the products as the reaction proceeded. However,as products Without are produced,there is a mixing further contribution to the Gibbs energy arising from their sqqis mixing (lowest curve) Including mixing The sum oftwo contributions has a minimum.That Extent of reaction, minimum corresponds to the equilibrium composition of the Mixing system. PDF文件使用"pdfFactory Pro”试用版本创建iw,fineprint.com,cn
If we did not have mixing of reactants and products, the Gibbs energy would change continuously from that of the reactants to that of the products as the reaction proceeded. However, as products are produced, there is a further contribution to the Gibbs energy arising from their mixing (lowest curve). The sum of two contributions has a minimum. That minimum corresponds to the equilibrium composition of the system. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì

General Expression for an Equilibrium Constant Ildeal Gases】 Consider a reaction in which there are more than one mole of reactants or products,e.g., N2(g)+3H(g)2NH3(g) 0r, A+3B2C The reaction Gibbs free energy is △Gm=2uc-4A-3E Recalling that u=uo+RT In(P/PO) △,Gm=2uc-uA-3u°g+2RTin(Pcpm)-RT In(PPO))-3RT In(Pg/po) So△Gm=△Gm'+RTIn{(PP)2M(PAP)(PB/P PDF文件使用"pdfFactory Pro”试用版本创建w,fineprint.com.c四
General Expression for an Equilibrium Constant (Ideal Gases) Consider a reaction in which there are more than one mole of reactants or products, e.g., N2 (g) + 3H2 (g) F 2NH3 (g) or, A + 3 B F 2 C The reaction Gibbs free energy is D rG m = 2 m C - m A - 3 mB Recalling that m = m o + RT ln( P/Po) DrGm= 2 m o C - m o A - 3 m o B + 2RT ln (PC /Po ) -RT ln (PA /Po ) -3RT ln(PB /Po ) So D rG m = D rG m o + RT ln{(PC /Po ) 2 /[(PA /Po )(PB /Po ) 3 ]} PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn Ì

When equilibrium is attained and the pressures are the equilibrium ones △,Gm°=-RTn9=-RTln{(Pc.eg/PI(Pa.eg/P)PB.ed/P)]} Perfect gas equilibria △,Gm=△,Gm+RTlnJp Vo-o8 D The ratio Jp is called the reaction quotient.The standard reaction Gibbs energy,A,Go is defined as the difference in the standard molar Gibbs energy of the reactants and products. PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com.c四
When equilibrium is attained and the pressures are the equilibrium ones DrG m o = - RT ln Ko = - RT ln{(PC,eq/Po ) 2 /[(PA,eq/Po )(PB,eq/Po ) 3 ]} DrGm =DrG0 m +RTlnJP Perfect gas equilibria ( ) B P B P P B J n =P The ratio JP is called the reaction quotient. The standard reaction Gibbs energy, DrG0 m is defined as the difference in the standard molar Gibbs energy of the reactants and products. PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn ÿ
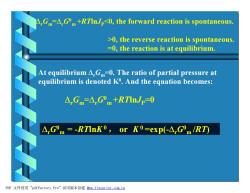
△,Gm△,G"m+RTInJp0,the forward reaction is spontaneous.. >0,the reverse reaction is spontaneous. =0,the reaction is at equilibrium. At equilibrium A.G=0.The ratio of partial pressure at equilibrium is denoted K.And the equation becomes: △Gm=△Gm+RTInJp=0 A.Gm=-RTInK0,or K0=exp(-AGOm/RT) PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,cn
DrGm =DrG0 m +RTlnJP0, the reverse reaction is spontaneous. =0, the reaction is at equilibrium. At equilibrium DrGm=0. The ratio of partial pressure at equilibrium is denoted K0 . And the equation becomes: DrGm =DrG0 m +RTlnJP=0 f f f f DrG0 m = -RTlnK0 , or K 0 =exp(-DrG0 m /RT) PDF 文件使用 "pdfFactory Pro" 试用版本创建 2www.fineprint.com.cn
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 4 The thermodynamics of mixtures.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 3 The Second Law of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 2 First Law Of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 1 The properties of gases.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 0 PYHSICAL CHEMISTRY(Introduction).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题化学动力学(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题表面胶体化学(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题统计热力学(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题相平衡(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题电化学(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题多组分热力学(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题热力学第二定律(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题热力学第一定律(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题气体pVT关系(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)物理化学(下册)提高题及参考答案(第7-11章).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)物理化学(上册)提高题及参考答案(第1-6章).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)2010-2011年第一学期物理化学(上册)期末试题.doc
- 北京化工大学:《物理化学》课程教学资源(作业习题)2009-2010年第一学期物理化学(上册)期末试题.doc
- 北京化工大学:《物理化学》课程教学资源(实验指导)治理工业含苯废气Cu-Mn-O复合氧化物催化剂的活性评价.pdf
- 北京化工大学:《物理化学》课程教学资源(实验指导)用差热分析方法测定催化剂的还原度及还原活化能.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 6 Phase Equilibrium.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 7 Electrochemistry.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 8 Elementary Statistical Thermodynamics.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 9 Surfaces(Interfacial Chemistry).pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 10 Chemical Reaction Kinetics.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 11 Colloid Chemistry.pdf
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第一章 绪论与气体pVT关系(性质)Physical Chemistry.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第三章 热力学第二定律 The second law of thermodynamics.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第二章 热力学第一定律 The first law of thermodynamics.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第四章 多组分系统热力学 Thermodynamics for open systems.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第九章 电化学 Electrochemistry.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第十章 界面化学 Surface Chemistry.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第五章 化学平衡 Chemical equilibrium.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第七章 统计热力学初步 Statistical Thermodynamics.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第六章 相平衡 Phase equilibrium.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第十一章 胶体化学 Colloid Chemistry.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第八章 化学反应动力学 Chemical reaction dynamics.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科非化工专业)第一章 绪论及气体的PVT性质 Physical Chemistry(主讲:张丽丹).pdf
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科非化工专业)第三章 热力学热二定律 The Second Law of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科非化工专业)第二章 热力学热一定律 The First Law of Thermodynamics.pdf
