北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 6 Phase Equilibrium

Phase Equilibrium 1 PDF文件使用"pdfFactory Pro”试用版本创建mm,fineprint.com,cn
1 Phase Equilibrium PDF 文件使用 "pdfFactory Pro" 试用版本创建 www.fineprint.com.cn

Introduction .Firstly,we introduce the famous phase rule of Gibbs which shows the extent to which various parameters can be varied yet the equilibrium between phases preserved. .With the rule established,we see how it can be used to discuss the phase diagrams.The chapter then introduces systems of gradually increasing complexity.In each case we shall see how the phase diagram for the system summarizes empirical observations on the conditions under which the various phases of the system are stable. 2 PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
2 Introduction •Firstly, we introduce the famous phase rule of Gibbs ,which shows the extent to which various parameters can be varied yet the equilibrium between phases preserved. •With the rule established, we see how it can be used to discuss the phase diagrams. The chapter then introduces systems of gradually increasing complexity. In each case we shall see how the phase diagram for the system summarizes empirical observations on the conditions under which the various phases of the system are stable. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn ÿ

Introduction .Why to learn this chapter? Phase diagrams are of considerable commercial and industrial significance,particularly for semiconductors, ceramics,steels,and alloys.They are also the basis of separation procedures in the petroleum industry and of the formulation of foods and cosmetic preparations. 3 PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,cn
3 Introduction •Why to learn this chapter? Phase diagrams are of considerable commercial and industrial significance, particularly for semiconductors, ceramics, steels, and alloys. They are also the basis of separation procedures in the petroleum industry and of the formulation of foods and cosmetic preparations. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn

Phase,components,and degrees of freedom All phase diagrams can be discussed in terms of a relationship,the phase rule,derived by Gibbs.We shall derive this rule first,and then apply it to a wide variety of systems.The phase rule requires a careful use of terms,so we begin by presenting a number of definitions Definitions Phases are the states of matter that is uniform throughout, not only in chemical composition but also in physical state. These comprise solid,liquid or gaseous (vapour). PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,c四
4 Phase, components, and degrees of freedom All phase diagrams can be discussed in terms of a relationship, the phase rule, derived by Gibbs. We shall derive this rule first,and then apply it to a wide variety of systems. The phase rule requires a careful use of terms, so we begin by presenting a number of definitions. Definitions Phases are the states of matter that is uniform throughout, not only in chemical composition but also in physical state. These comprise solid, liquid or gaseous (vapour). PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn

The number of phases in a system is denoted P (a)A gas,or a gaseous mixture is a single phase. P=1 (b)For a solid system,an alloy of two metals is a two-phase system(P=2)if the metals are immiscible,but a single-phase system(P=1)if they are miscible---a homogeneous mixture of the two substances---is uniform on a molecular scale. 5 PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com,cn
5 The number of phases in a system is denoted P (a)A gas, or a gaseous mixture is a single phase. P=1 (b) For a solid system, an alloy of two metals is a two-phase system(P=2) if the metals are immiscible, but a single-phase system(P=1) if they are miscible---a homogeneous mixture of the two substances---is uniform on a molecular scale. PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn

(c)For a liquid system,according to the solubility to decide whether a system consists of one phase or of two.For example,a solution of sodium chloride in water is a single phase.A pair of liquids that are partially miscible is a two-phase system(P=2) CaCO3(s)CaO(s)+CO,(g) Phase 1 Phase 2 Phase 3 6 PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn
6 (c) For a liquid system, according to the solubility to decide whether a system consists of one phase or of two. For example, a solution of sodium chloride in water is a single phase. A pair of liquids that are partially miscible is a two-phase system(P=2) CaCO3 (s) F CaO(s) + CO2 (g) Phase 1 Phase 2 Phase 3 PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn
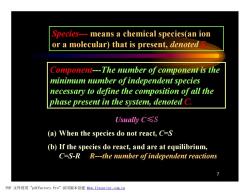
Species---means a chemical species(an ion or a molecular)that is present,denoted Component---The number of component is the minimum number ofindependent species necessary to define the composition of all the phase present in the system,denoted C. Usually C≤S (a)When the species do not react,C=S (b)If the species do react,and are at equilibrium, C=S-R R---the number of independent reactions PDF文件使用"pdfFactory Pro”试用版本创建ww,fineprint.com,cn
7 Species--- means a chemical species(an ion or a molecular) that is present, denoted S. Component---The number of component is the minimum number of independent species necessary to define the composition of all the phase present in the system, denoted C. Usually C≤S (a) When the species do not react, C=S (b) If the species do react, and are at equilibrium, C=S-R R---the number of independent reactions PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn

(c)The limited concentration in the same phase R'---the number of concentration limitation C=S-R-R' Note:S can be different according to your opinion,but C is only one for a given system. Degrees of freedom---is the number ofintensive variables that can be changed independently without disturbing the number of phases in equilibrium.denoted F. 8 PDF文件使用"pdfFactory Pro”试用版本创建m,fineprint.com,cn
8 (c) The limited concentration in the same phase R’---the number of concentration limitation C=S-R-R’ Degrees of freedom---is the number of intensive variables that can be changed independently without disturbing the number of phases in equilibrium. denoted F. Note: S can be different according to your opinion, but C is only one for a given system. PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn

The phase rule J.W.Gibbs deduced the phase rule,which is a general relation between the variance,F,the number of component,C,and the number of phases at equilibrium,P,for a system of any composition: F=C-P+2 9 PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint.com,cn
9 The phase rule J.W. Gibbs deduced the phase rule, which is a general relation between the variance, F, the number of component, C,and the number of phases at equilibrium ,P, for a system of any composition: F=C-P+2 PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn

Deduce the phase rule F=the total number of intensive variables the total number ofrelations Suppose:there are S species in every phase for P Phases Intensive variables:T,P,count as 2;there are S species in every phase for P Phases,so the total number of intensive variables is PS+2. Relations:because Exg=1 in every phase and there is P phases and He(1)=uB(2)=g(3)--B(P),the total number is S(P-1);there are R independent reactions and R'concentration limitations the total number of relations is P+S(P-1)+R+R So F=(PS+2)-/P+S(P-1)+R+RJ=S-R-R-P+2 F=C-P+2 10 PDF文件使用"pdfFactory Pro”试用版本创建wmw,fineprint.com,c四
10 Deduce the phase rule F=the total number of intensive variables-the total number of relations Suppose:there are S species in every phase for P Phases Intensive variables:T,P,count as 2; there are S species in every phase for P Phases,so the total number of intensive variables is PS+2. Relations:because ∑xB =1 in every phase and there is P phases ;and mB (1)=mB (2)=mB (3)=┄= mB (P),the total number is S(P-1);there are R independent reactions and R¢ concentration limitations the total number of relations is P+S(P-1)+R+R¢ So F=(PS+2)-[P+S(P-1)+R+R¢]=S-R-R¢-P+2 F=C-P+2 PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 5 Chemical Equilibrium.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 4 The thermodynamics of mixtures.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 3 The Second Law of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 2 First Law Of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 1 The properties of gases.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 0 PYHSICAL CHEMISTRY(Introduction).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题化学动力学(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题表面胶体化学(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题统计热力学(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题相平衡(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题电化学(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题多组分热力学(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题热力学第二定律(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题热力学第一定律(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)计算题气体pVT关系(含参考答案).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)物理化学(下册)提高题及参考答案(第7-11章).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)物理化学(上册)提高题及参考答案(第1-6章).pdf
- 北京化工大学:《物理化学》课程教学资源(作业习题)2010-2011年第一学期物理化学(上册)期末试题.doc
- 北京化工大学:《物理化学》课程教学资源(作业习题)2009-2010年第一学期物理化学(上册)期末试题.doc
- 北京化工大学:《物理化学》课程教学资源(实验指导)治理工业含苯废气Cu-Mn-O复合氧化物催化剂的活性评价.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 7 Electrochemistry.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 8 Elementary Statistical Thermodynamics.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 9 Surfaces(Interfacial Chemistry).pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 10 Chemical Reaction Kinetics.pdf
- 北京化工大学:《物理化学》课程电子教案(理科双语)Chapter 11 Colloid Chemistry.pdf
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第一章 绪论与气体pVT关系(性质)Physical Chemistry.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第三章 热力学第二定律 The second law of thermodynamics.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第二章 热力学第一定律 The first law of thermodynamics.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第四章 多组分系统热力学 Thermodynamics for open systems.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第九章 电化学 Electrochemistry.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第十章 界面化学 Surface Chemistry.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第五章 化学平衡 Chemical equilibrium.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第七章 统计热力学初步 Statistical Thermodynamics.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第六章 相平衡 Phase equilibrium.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第十一章 胶体化学 Colloid Chemistry.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科化工专业)第八章 化学反应动力学 Chemical reaction dynamics.pps
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科非化工专业)第一章 绪论及气体的PVT性质 Physical Chemistry(主讲:张丽丹).pdf
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科非化工专业)第三章 热力学热二定律 The Second Law of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科非化工专业)第二章 热力学热一定律 The First Law of Thermodynamics.pdf
- 北京化工大学:《物理化学》课程电子教案(PPT教学课件,工科非化工专业)第七章 化学反应动力学 Chemical Reaction Dynamics.pdf
