安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(课件讲稿,英文版)新药研发 New drugs development(主讲:(张玲玲)

New drugs development 新药研发 Professor Lingling Zhang(张玲玲) Institute of clinical pharmacology,AHMU 安徽医科大学临床药理研究所) Izhang@ahmu.edu.cn
New drugs development 新药研发 Professor Lingling Zhang (张玲玲 ) Institute of clinical pharmacology, AHMU (安徽医科大学临床药理研究所 ) llzhang@ahmu.edu.cn

Outlines I.Preclinical development of new drugs; II.Clinical trial of new drugs: 1.GCP and the significance of implementing GCP. 2.International Council for Harmonization,ICH 3.Phase I clinical trial tolerance test and pharmacokinetic studies. 4.Phase II clinical trial. 5.Phase III clinical trial. 6.Phase IV clinical trial. 7.Drug bioequivalence test. III.Drug consistency evaluation 2019/8/28 Institute of clinical pharmacology,AHMU 2
Outlines I. Preclinical development of new drugs; II. Clinical trial of new drugs: 1.GCP and the significance of implementing GCP. 2. International Council for Harmonization, ICH 3. PhaseⅠ clinical trial : tolerance test and pharmacokinetic studies. 2019/8/28 Institute of clinical pharmacology, AHMU 2 4. Phase II clinical trial. 5. Phase III clinical trial. 6. Phase IV clinical trial. 7. Drug bioequivalence test. III. Drug consistency evaluation

Aims 1.To know of: (1)The definition of drugs,new drug and bioavailability. (2)The components of preclinical drug development. 2.To master (1)The definition of GCP. (2)The determination of minimum first dose. (3)The purpose and significance Phase II clinical trial. (4)The purpose and significance of human bioavailability test. 2019/8/28 Institute of clinical pharmacology,AHMU 3
Aims 1. To know of : (1) The definition of drugs, new drug and bioavailability. (2) The components of preclinical drug development. 2. To master : (1) The definition of GCP . 2019/8/28 Institute of clinical pharmacology, AHMU 3 (2) The determination of minimum first dose. (3) The purpose and significance Phase II clinical trial. (4) The purpose and significance of human bioavailability test
A example: Headache Drug Alleviation Question:What's drug? 2019/8/28 Institute of clinical pharmacology,AHMU 4
A example: Headache Drug Alleviation 2019/8/28 Institute of clinical pharmacology, AHMU 4 Headache Drug Alleviation Question: What’s drug?

The definition of drugs and new drugs 1.Drugs:drugs are some materials,which are used for the prevention,treatment and diagnosis of diseases,regulating physiological function,and having indications,major functions and usage. 2019/8/28 Institute of clinical pharmacology,AHMU 5
The definition of drugs and new drugs 1.Drugs: drugs are some materials, which are used for the prevention, treatment and diagnosis of diseases, regulating physiological function, and having indications, major functions and usage. 2019/8/28 Institute of clinical pharmacology, AHMU 5

2.New drugs:new drugs are the drugs that are not posted in state pharmacopoeia()or approved to be used by the state department of pharmaceutical administration. (National Medical Products Administration,NMPA) 国家药品监督管理局 National Medical Products Administration 中华人民共和国 药典 通想 木神机由 +中4aa。 2019/8/28 Institute of clinical pharmacology,AHMU 6
2.New drugs: new drugs are the drugs that are not posted in state pharmacopoeia(药典) or approved to be used by the state department of pharmaceutical administration. (National Medical Products Administration, NMPA) 2019/8/28 Institute of clinical pharmacology, AHMU 6

I Preclinical development of new drugs 2019/8/28 Institute of clinical pharmacology,AHMU
I Preclinical development of new drugs 2019/8/28 Institute of clinical pharmacology, AHMU 7
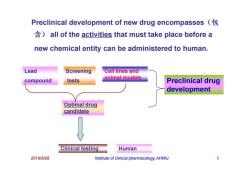
Preclinical development of new drug encompasses all of the activities that must take place before a new chemical entity can be administered to human. Lead Screening Cell lines and compound tests animal models Preclinical drug development Optimal drug candidate Clinical testing Human 2019/8/28 Institute of clinical pharmacology,AHMU 8
Lead compound Screening tests Cell lines and animal models Preclinical drug development Preclinical development of new drug encompasses(包 含) all of the activities that must take place before a new chemical entity can be administered to human. 2019/8/28 Institute of clinical pharmacology, AHMU 8 Optimal drug candidate Clinical testing development Human

Generally,preclinical drug development includes three areas (1)Vitro studies,药理学特性 (2)Drug supply and formulation (3)Vivo studies in animal models. 治疗原则证据和蘑在临床疗减 2019/8/28 Institute of clinical pharmacology,AHMU 9
Generally, preclinical drug development includes three areas : (1) Vitro studies, 药理学特性 (2) Drug supply and formulation , (3) Vivo studies in animal models. 2019/8/28 Institute of clinical pharmacology, AHMU 9 治疗原则证据和潜在临床疗效

1.In Vitro Studies (1)Vitro screening assays are to study the ability of drugs: To bind to receptors, To inhibit specific enzymes, To stabilize or destabilize multi protein complexes. URP "Looks like Carstairs finally got a single cell to eat a whole meal By the way,have you seen Carstairs lately?" 2019/8/28 Institute of clinical pharmacology,AHMU 10
1. In Vitro Studies (1) Vitro screening assays are to study the ability of drugs: To bind to receptors, To inhibit specific enzymes, To stabilize or destabilize multi protein complexes. 2019/8/28 Institute of clinical pharmacology, AHMU 10
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(课件讲稿,英文版)药物作用评价 Assessment of drug effects(主讲:汪庆童).pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(课件讲稿,英文版)临床药理学概论 Introduction to clinlcal pharmacology(主讲:魏伟).pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)New drugs development.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Charpter 24 Drug therapy in the elderly.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Charpter 23 Drug therapy in neonates and pediatric patients.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Charpter 22 Drug therapy in pregant and nursing women.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Time Course of Drug Response.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Dose-effect and concentration-effect analysis.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Physiological and laboratory markers of drug effect.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Chapter 16 Drug Toxicity and Adverse Drug Reactions.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Chapter 15 Drug Interactions.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Chapter 13.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Chapter One.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(授课教案,英文版)Chapter One Introduction to Clinical Pharmacology.pdf
- 江苏第二师范学院:一种用于制药压片生产的虚拟仿真平台.pdf
- 安徽医科大学:《药剂学》课程教学资源(各章作业习题,含参考答案).pdf
- 安徽医科大学:《药剂学》课程教学资源(教学大纲).pdf
- 长治医学院:《药理学》课程思政融入实践.pdf
- 电子科技大学医学院:《高级临床药学实践 Advanced clinical pharmaceutical practice》课程教学资源(课件讲稿)18 新冠肺炎防控中药学专业担当.pdf
- 电子科技大学医学院:《高级临床药学实践 Advanced clinical pharmaceutical practice》课程教学资源(课件讲稿)17 抗菌药物PK、PD与临床合理使用 Antibacterial PK/PD and rational clinical use.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(课件讲稿,英文版)Clinical Pharmacogenetics Drug interaction Drug toxicology(主讲:常艳).pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(课件讲稿,英文版)Clinical Pharmacokinetics.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(课件讲稿,英文版)新生儿及儿童合理用药 Drug Therapy in Neonates and children.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(课件讲稿,英文版)特殊人群的药物治疗学 Drug Therapy in Pregnant.pdf
- 安徽医科大学:《临床药理学 Clinical Pharmacology》课程教学资源(课件讲稿,英文版)老年人药物治疗 Drug therapy in the elderly.pdf
- 温州医科大学:《药理学》课程教学资源(练习题,人卫专科八版,含答案).pdf
- 温州医科大学:临床医学专业《药理学》课程教学资源(练习资料,人卫九版,含答案).doc
- 温州医科大学:《药理学》课程教学资源(护理学专业,练习资料,人卫九版,含答案).doc
- 甘肃农业大学:《药用植物栽培学》课程教学资源(教学大纲,打印版).pdf
- 甘肃农业大学:《药用植物栽培学》课程教学资源(理论教案,打印版).pdf
- 甘肃农业大学:《药用植物栽培学》课程教学资源(实验教案,打印版).pdf
- 西南交通大学:《制药工艺学 Pharmaceutical Technology》课程教学资源(课件讲稿)化学制药工艺安全性(主讲:陆群).pdf
- 西南交通大学:《制药工艺学 Pharmaceutical Technology》课程教学资源(课件讲稿)生物制药工艺——青霉素的发酵生产工艺.pdf
- 西南交通大学:《制药工艺学 Pharmaceutical Technology》课程教学资源(课件讲稿)重组人干扰素生产工艺.pdf
- 西南交通大学:《制药工艺学 Pharmaceutical Technology》课程教学资源(课件讲稿)奥美拉唑的生产工艺原理.pdf
- 上海中医药大学:课程教学大纲汇编合集(2017年版)教学大纲(外语中心).pdf
- 河南大学:《药理学》课程教学资源(教学大纲)药理学教学大纲(理论).pdf
- 河南大学:《药理学》课程教学资源(讲义)第一章 药理学总论——绪言.pdf
- 河南大学:《药理学》课程教学资源(讲义)第二章 药物效应动力学.pdf
- 河南大学:《药理学》课程教学资源(讲义)第三章 药物代谢动力学.pdf
