上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 16_Control volume analysis - mass conservation

上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lectures 16 Chapter 5 Mass and Energy Analysis of Control Volume Analysis Spring,3/28/2018 Prof.,Dr.Yonghua HUANG 强 。 http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lectures 16 Spring, 3/28/2018 Prof., Dr. Yonghua HUANG Chapter 5 Mass and Energy Analysis of Control Volume Analysis http://cc.sjtu.edu.cn/G2S/site/thermo.html
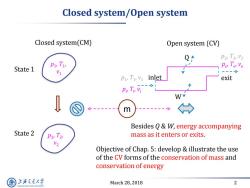
Closed system/Open system Closed system(CM) Open system (CV) P2,T2,V2 T State 1 ---> pT,v inlet exit Pr Ty Vi m Besides 0&W,energy accompanying State 2 T2 mass as it enters or exits. Objective of Chap.5:develop illustrate the use of the CV forms of the conservation of mass and conservation of energy 上游究通大学 March 28,2018 2 SHANGHAI JLAO TONG UNIVERSITY
March 28, 2018 2 Closed system/Open system Closed system(CM) Open system (CV) State 1 State 2 p1 , T1 , v1 p2 , T2 , v2 m p1 , T1 , v1 p2 , T2 , v2 inlet exit Objective of Chap. 5: develop & illustrate the use of the CV forms of the conservation of mass and conservation of energy Q W Besides Q & W, energy accompanying mass as it enters or exits. pi , Ti , vi pe , Te , ve
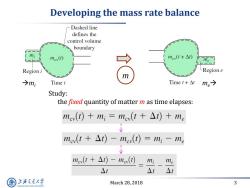
Developing the mass rate balance Dashed line defines the control volume boundary mi nev(①) nev(t+△) Region i LRegion e m →m; Time t Time t+△t me→ Study: the fixed quantity of matter m as time elapses: mev(t)+mi=mev(t At)+me mev(t At)-moy(t)=mi-me me(t+△t)-nev(t) mi me △t △t△t 上游充通大 March 28,2018 3 SHANGHAI JIAO TONG UNIVERSITY
March 28, 2018 3 Developing the mass rate balance Study: the fixed quantity of matter m as time elapses: mi me m
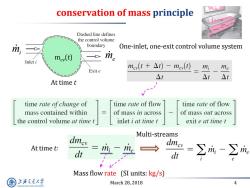
conservation of mass principle mn=0 Dashed line defines the control volume mi boundary One-inlet,one-exit control volume system mev(t) > me Inlet i mcv(t+△t)-mev(t) mi me Exit e △t △t △t At time t time rate of change of time rate of flow time rate of flow mass contained within of mass in across of mass out across the control volume at time t inlet i at time t exit e at time t Multi-streams dmev At time t: dt mi me → Mass flow rate (SI units:kg/s) 上游充通大 March 28,2018 4 SHANGHAI JIAO TONG UNIVERSITY
March 28, 2018 4 conservation of mass principle At time t mcv(t) mi me At time t: Mass flow rate (SI units: kg/s) Multi-streams One-inlet, one-exit control volume system
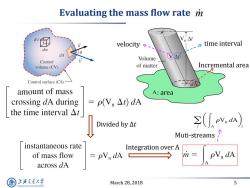
Evaluating the mass flow rate mi velocitys、 time interval dm dA Volume Control volume (CV) of matter Incremental area Control surface (CS) amount of mass A:area crossing dA during =pVn△t)dA the time interval△t Divided by△t ≥(N.a) Muti-streams instantaneous rate Integration over A of mass flow =pVn dA 防= PVn dA across dA 上究大学 March 28,2018 5 SHANGHAI JIAO TONG UNIVERSITY
March 28, 2018 5 Evaluating the mass flow rate m velocity time interval : area Incremental area Divided by ∆t Integration over A Muti-streams
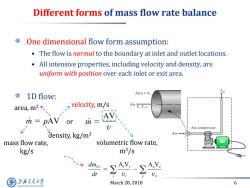
Different forms of mass flow rate balance One dimensional flow form assumption: The flow is normal to the boundary at inlet and outlet locations. All intensive properties,including velocity and density,are uniform with position over each inlet or exit area. 1 D flow: Area=A area,m2、 velocity,m/s Air V.T.v m=pAV or Air compressor density,kg/m3 Air mass flow rate, volumetric flow rate, kg/s m3/s dt U: 上游充通大学 March 28,2018 6 SHANGHAI JLAO TONG UNIVERSITY
March 28, 2018 6 Different forms of mass flow rate balance One dimensional flow form assumption: • The flow is normal to the boundary at inlet and outlet locations. • All intensive properties, including velocity and density, are uniform with position over each inlet or exit area. 1D flow: or velocity, m/s area, m2 density, kg/m3 mass flow rate, kg/s volumetric flow rate, m3/s
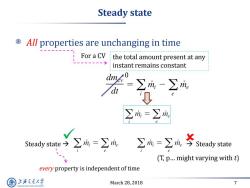
Steady state All properties are unchanging in time For a CV the total amount present at any instant remains constant ∑=∑m Steady sate今∑m=∑m】 ∑m=∑成芩Sady stae (T,p...might varying with t) every property is independent of time 上游充通大 March 28,2018 7 SHANGHAI JLAO TONG UNIVERSITY
March 28, 2018 7 Steady state All properties are unchanging in time For a CV the total amount present at any instant remains constant Steady state Steady state (T, p… might varying with t) every property is independent of time 0
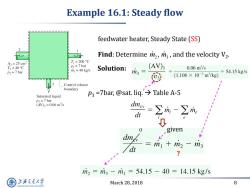
Example 16.1:Steady flow feedwater heater,Steady State (SS) b Find:Determine mz,m,and the velocity V2. A2=25cm2 T1=200C T2=40C P1=7 bar rin =40 kg/s Solution: (AV)3 0.06m3/s P2=7 bar m3= (1.108×10-3m3/kg) =54.15kg/s 3 个 Control volume boundary Saturated liquid P3 =7bar,@sat.liq.>Table A-5 P3 7 bar (AV)3=0.06m3/s =∑m一∑m: dt 0 given dm dt m1+m2一m3 ? m2=m3-m1=54.15-40=14.15kg/s 上游充通大 March 28,2018 8 SHANGHAI JIAO TONG UNIVERSITY
March 28, 2018 8 Example 16.1: Steady flow feedwater heater, Steady State (SS) Find: Determine , , and the velocity V2 . m2 m3 Solution: ? p3 =7bar, @sat. liq. Table A-5 given
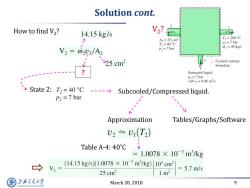
Solution cont. How to find V2? 14.15kg/s T=200C 1 A2=25cm2 T2=40C P1=7bar P2=7bar rin =40 kg/s V2=m22/A2 25cm2 Control volume boundary Saturated liquid P3=7bar (AV)3=0.06m3/s > State2:T2=40C----> Subcooled/Compressed liquid. P2 7 bar Approximation Tables/Graphs/Software U2≈U(T2) Table A-4:40°C =1.0078×10-3m3/kg (14.15kg/s)(1.0078×10-3m/kg)104cm2 V2= 5.7m/s 25cm2 1m2 上游充通大 March 28,2018 9 SHANGHAI JIAO TONG UNIVERSITY
March 28, 2018 9 Solution cont. ? State 2: Subcooled/Compressed liquid. Approximation Tables/Graphs/Software Table A-4: 40˚C How to find V2 ? V2 ?

Example 16.2 Transient problem open! Air Initially filled with water pulled out discharge plug near the bottom Water ·average velocity V=√2gh h is the height of water in the tank measured from the center of the hole (a variable) 121.92cmh07 g is the gravitational acceleration,=9.81m/s2 1.27cm Determine: e how long it will take for the water level in the tank to drop to ho/2 from the bottom? 0 Dtank 91.44cm Assumptions: [Textbook Example 5-2] 1.Water is an incompressible substance. 2.The distance between the bottom of the tank and the center of the hole is negligible compared to the total water height. Analysis: take the volume occupied by water as the CV---a variable CV an unsteady-flow problem since the properties within the CV change with time. 上降文通大学 March 28,2018 10 SHANGHAI JIAO TONG UNIVERSITY
March 28, 2018 10 Example 16.2 Transient problem 121.92cm 91.44 cm open 1.27 cm Determine: how long it will take for the water level in the tank to drop to h0/2 from the bottom? Assumptions: 1. Water is an incompressible substance. 2. The distance between the bottom of the tank and the center of the hole is negligible compared to the total water height. Analysis: • take the volume occupied by water as the CV--- a variable CV • an unsteady-flow problem since the properties within the CV change with time. [Textbook Example 5-2]
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 15_Polytropic process.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 14_cv, cp, Δu, Δh of ideal gas and applied to close system.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 13_Equation of state and ideal gas model.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 12_Evaluating u, h, cp, cv properties.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 11_Retrieving pvt properties.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 09-10_Substance, property and phase.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 07-08_Energy balance for close system and cycles.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 05-06_Energy, work, heat transfer.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 03-04_Concepts.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 01-02_Course Introduction.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)中意楼位置.pptx
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 48_Review and Final Exam.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 47_Compressor, compression with intercooling.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 46_Diesel cycle and dual cycle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 45_Air standard cycle, internal combustion engines, Otto cycle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 44_Vapor-compression refrigeration, Heat pump systems.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 43_superheat and reaheat.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 39-40_vapor power cycles.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 37-38_Concept of exergy and apply to CM&CV systems.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 36_Heat transfer and Work of internal reversible, ss flow.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 17_Control volume analysis - energy conservation.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 18_Illustrations_1 Nozzles, diffusers, turbines.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 19_Illustrations_2 Compressors, pumps.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 20-21_Illustrations_3 Heat exchangers, throttling devices, System integration.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 22_Transient analysis of Energy.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 23-24_Introducing 2nd law, concept of irreversibilities.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 27-28_Applying 2nd law to thermodynamic cycles, Maximum performance.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 29_Carnot Cycle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 30_Clausius inequality and Entropy.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 31_Retrieve entropy data.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 32_Internally reversible processes, Closed system entropy balance.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 33_Entropy increase principle.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 34_Entropy balance to open systems.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 35_Isentropic processes, Isentropic efficiencies.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 36_Heat transfer and Work of internal reversible, ss flow.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 37_Concept of exergy and apply to CM systems.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 38_Exergy of CV systems.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 39-40_vapor power cycles.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 41-42_superheat and reaheat.pdf
- 上海交通大学:《热力学 Thermodynamics(I)》课程教学资源(课件讲义)Lecture 43-44_Vapor-compression refrigeration, Heat pump systems.pdf
