《动物免疫学》课程教学资源(文献资料)Antibody Structure
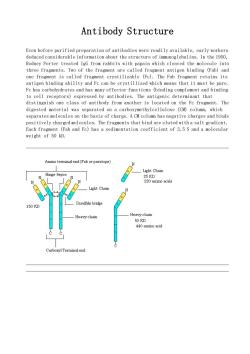
Antibody Structure Even before purified preparation of antibodies were readily available,early workers deduced considerable information about the structure of immunoglobulins.In the 1950, Rodney Porter treated IgG from rabbits with papain which cleaved the molecule into three fragments.Two of the fragment are called fragment antigen binding (Fab)and one fragmont is called fragment crystilizable (Fe).The Fab fragment retains its antigen binding ability and Fc can be crystillized which means that it must be pure. Fc has carbohydrates and has many effector functions (binding complement and binding to cell receptors)expressed by antibodies.The antigenic determinant that distinguish one class of antibody from another is located on the Fc fragment.The digested material was separated on a carboxymethylcellulose (CM)column,which separates molecules on the basis of charge.A CM column has negative charges and binds positively charged molecules.The fragments that bind are eluted with a salt gradient. Each fragment (Fab and Fc)has a sedimentation coefficient of 3.5 S and a molecular weight of 50 kD. Amino terminalend(Fab or paratope) Hinge Region Light Chain Light Chain Disulfide bridge 50D ainal end
Antibody Structure Even before purified preparation of antibodies were readily available, early workers deduced considerable information about the structure of immunoglobulins. In the 1950, Rodney Porter treated IgG from rabbits with papain which cleaved the molecule into three fragments. Two of the fragment are called fragment antigen binding (Fab) and one fragment is called fragment crystilizable (Fc). The Fab fragment retains its antigen binding ability and Fc can be crystillized which means that it must be pure. Fc has carbohydrates and has many effector functions (binding complement and binding to cell receptors) expressed by antibodies. The antigenic determinant that distinguish one class of antibody from another is located on the Fc fragment. The digested material was separated on a carboxymethylcellulose (CM) column, which separates molecules on the basis of charge. A CM column has negative charges and binds positively charged molecules. The fragments that bind are eluted with a salt gradient. Each fragment (Fab and Fc) has a sedimentation coefficient of 3.5 S and a molecular weight of 50 kD
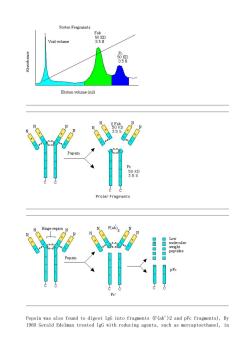
Porter Fregments Void volume (ml) Proter Fragments Hinge region F(ab) Pepsin was also found to digest IgG into fragments (F(ab')2 and pFc fragments).By 1960 Gerald Edelman treated IgG with reducing agents,such as mercaptoethanol,in
Pepsin was also found to digest IgG into fragments (F(ab')2 and pFc fragments). By 1960 Gerald Edelman treated IgG with reducing agents, such as mercaptoethanol, in
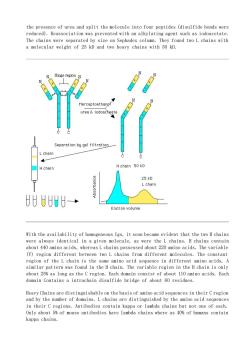
the presence of urea and split the molecule into four peptides (disulfide bonds were reduced).Reassociation was prevented with an alkylating agent such as iodoacetate. Hinge region】 urea&iodoategtg Separation by gel filtretion L chain Hchain Hchain 50 kD 25 kD U8→ Elution volume With the availability of homogeneous Igs,it soon became evident that the two H chains were always identical in a given molecule,as were the L chains.H chains contain about 440 amino acids,whereasLchains possessed about 220 amino acids.The variable (V)region different between two L chains from different molecules.The constant region of the L chain is the same amino acid sequence in different amino acids.A similar pattern was found in the H chain.The variable region in the H chain is only about 25%as long as the C region.Each domain consist of about 110 amino acids.Each domain Contains a intrachain disulfide bridge of about 60 residues. Heavy Chains are distinguishable on the basis of amino acid sequences in their Cregion and by the number of domains.L chains are distinguished by the amino acid sequences in their C regions.Antibodies contain kappa or lambda chains but not one of each. Only about 5%of mouse antibodies have lambda chains where as 40%of humans contain kappa chains
the presence of urea and split the molecule into four peptides (disulfide bonds were reduced). Reassociation was prevented with an alkylating agent such as iodoacetate. The chains were separated by size on Sephadex column. They found two L chains with a molecular weight of 25 kD and two heavy chains with 50 kD. With the availability of homogeneous Igs, it soon became evident that the two H chains were always identical in a given molecule, as were the L chains. H chains contain about 440 amino acids, whereas L chains possessed about 220 amino acids. The variable (V) region different between two L chains from different molecules. The constant region of the L chain is the same amino acid sequence in different amino acids. A similar pattern was found in the H chain. The variable region in the H chain is only about 25% as long as the C region. Each domain consist of about 110 amino acids. Each domain Contains a intrachain disulfide bridge of about 60 residues. Heavy Chains are distinguishable on the basis of amino acid sequences in their C region and by the number of domains. L chains are distinguished by the amino acid sequences in their C regions. Antibodies contain kappa or lambda chains but not one of each. Only about 5% of mouse antibodies have lambda chains where as 40% of humans contain kappa chains

Human Kappa chain has about four subgroups,while lambda has about six sub groups. Each subgroup contains certain amino acids in defined positions that are never changed, while other amino acids that localize nearby may display pronounced variability Light Chain Variable Regions Hypervariable subregions were observed in three sites within Kappa and lambda variable subregions.Each hyper variable region contains about 10 amino acids,which serve as contact residues for antibody binding.They function as complementary determining regions (CDRs)with respect to the binding of an antigen.The three hypervariable sub regions (CDRs)of the L chain and the CDRs on the H chain determine antibody specificity for antigen.In the variable region there are areas that are fairly constant in between the CDRs. Index of variability Measurement of variability at any amino acid position may be calculated by the following equation. Variability =Number of different amino acids found at that point/Frequency of the most common amino acid at that position Thus,the index can range from 1,for a completely invariant position,to a maximum of 400 for a position in which all 20 amino acids are different. y=1/1=1 V=20/(1/20)=400
Human Kappa chain has about four subgroups, while lambda has about six sub groups. Each subgroup contains certain amino acids in defined positions that are never changed, while other amino acids that localize nearby may display pronounced variability Light Chain Variable Regions Hypervariable subregions were observed in three sites within Kappa and lambda variable subregions. Each hyper variable region contains about 10 amino acids, which serve as contact residues for antibody binding. They function as complementary determining regions (CDRs) with respect to the binding of an antigen. The three hypervariable sub regions (CDRs) of the L chain and the CDRs on the H chain determine antibody specificity for antigen. In the variable region there are areas that are fairly constant in between the CDRs. Index of variability Measurement of variability at any amino acid position may be calculated by the following equation. Variability = Number of different amino acids found at that point/Frequency of the most common amino acid at that position. Thus, the index can range from 1, for a completely invariant position, to a maximum of 400 for a position in which all 20 amino acids are different. V = 1/1 =1 V= 20/(1/20) = 400
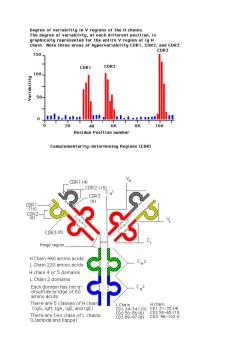
Degree of variability in v regions of the H chains. gree of var chain.Note three areas of hypervariability CDR1,CDR2,and CDR3. DR. 150 CDR1 CDR2 100 .....1.......mm 0 40 60 80 100 Residue Position number Complementarity-determining Regions (CDR) CDR1(4) _CDR2 (15 DR3 (8) Hinge region HChain 440 amino acids L Chain 220 amino acid H chain 4 or 5 domains L Chain 2 domains 5achaeenge6 5 amino acids. There are 5 classes of H chai (IgG,IgM,IgA,IgD,and IgE) (Limchains
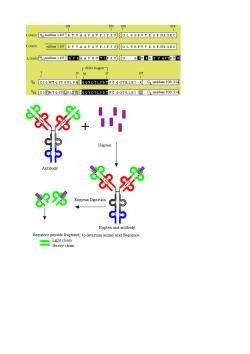
120200 chain风is107RAAP名FIFPCL8名PyK名NRGEG chain sidues107 BTVAAP名VF1FP卫GL名名PyTK名F NRGEG chain及3 resies1*107 QP KAAP名TF卫FPPg.·名团V EK T V AP证C图 107 W DIQMTQSP SSLBR QQTDELPBIFG QGTKLEI-K CLride 108.214 冬%+出 Hapten Antibody 汉 83% Hapten and antibody Sequence peptide frgmen to determina Sequence Light chain Heavy chan
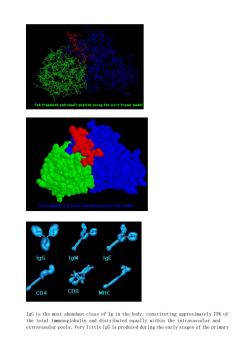
Fab fregment and small peptide using the vire frame mode CD4 CD8 IgG is the most abundant class of Ig in the body,constituting approximately 75%of the total immunoglobulin and distributed equally within the intravascular and extravasular pools.Very little IgG is produced during the early stages of the primary
IgG is the most abundant class of Ig in the body, constituting approximately 75% of the total immunoglobulin and distributed equally within the intravascular and extravasular pools. Very little IgG is produced during the early stages of the primary
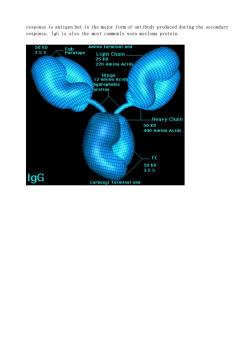
response to antigen but is the major form of antibody produced during the secondary response.IgG is also the most commonly seen myeloma protein. 50 5atog Amino terminal end Light Chain_ 220 Amino Acid ydrophobic Heavy Chair -FC 98 IgG end
response to antigen but is the major form of antibody produced during the secondary response. IgG is also the most commonly seen myeloma protein
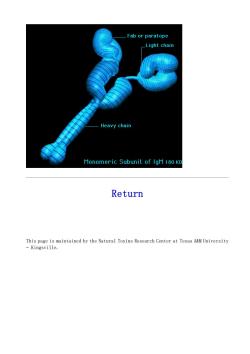
Fab or paratope _Light chain Heavy chain Monomeric Subunit of IgM 180 K Return This page is maintained by the Natural Toxins Research Center at Texas A&M University -Kingsville
Return This page is maintained by the Natural Toxins Research Center at Texas A&M University - Kingsville
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 《动物免疫学》课程教学资源(文献资料)Antibody Production 2.doc
- 《动物免疫学》课程教学资源(文献资料)Antibody Production 1.doc
- 《动物免疫学》课程教学资源(文献资料)Acquired Immune Deficiency Syndrome 1.doc
- 《动物免疫学》课程教学资源(文献资料)Acquired Immune Deficiency Syndrome 2.doc
- 广东海洋大学:《动物免疫学》课程电子教案(PPT教学课件,完整讲稿,共十三章,主讲:徐春厚).ppt
- 中国农业出版社:《兽医免疫学》课程教学资源(书籍教材)兽医免疫学 veterinary immunology(PDF电子版,共二十五章,主编:崔治中、崔保安).pdf
- 广东海洋大学:《动物免疫学》授课教案(打印版)免疫学 Immunology(生物技术专业).pdf
- 广东海洋大学:《动物免疫学》授课教案(打印版)动物免疫学 Animal Immunology(动物医学专业).pdf
- 广东海洋大学:《动物免疫学》课程实验指导(打印版)动物免疫学实验指导(第二版).pdf
- 广东海洋大学:《动物免疫学》课程教学课件(打印版)动物免疫学各章讲义(共十三章).pdf
- 上海交通大学:动物科学专业《动物解剖学》课程教学大纲.pdf
- 上海交通大学:动物科学专业《生物统计与试验设计》课程教学大纲.pdf
- 上海交通大学:动物科学专业《微生物学(E类)》课程教学大纲.pdf
- 上海交通大学:动物科学专业《动物生理学》课程教学大纲.pdf
- 上海交通大学:动物科学专业《动物学》课程教学大纲.pdf
- 上海交通大学:动物科学专业《生态学》课程教学大纲.pdf
- 上海交通大学:动物科学专业《动物生物技术》课程教学大纲.pdf
- 上海交通大学:动物科学专业《动物免疫学》课程教学大纲.pdf
- 上海交通大学:动物科学专业《动物营养学》课程教学大纲.pdf
- 上海交通大学:动物科学专业《分子生物学》课程教学大纲.pdf
- 《动物免疫学》课程教学资源(文献资料)Cell Mediated Immunity.doc
- 《动物免疫学》课程教学资源(文献资料)Cells and Tissue of the Immune Response.doc
- 《动物免疫学》课程教学资源(文献资料)Cells, Tissue, and Organs of the Immune System.doc
- 《动物免疫学》课程教学资源(文献资料)Classes of Antibodies.doc
- 《动物免疫学》课程教学资源(文献资料)Complement.doc
- 《动物免疫学》课程教学资源(文献资料)complement(有图).doc
- 《动物免疫学》课程教学资源(文献资料)ELISA技术.doc
- 《动物免疫学》课程教学资源(文献资料)Histocompatibility.doc
- 《动物免疫学》课程教学资源(文献资料)Humoral Response, Miguel Perez III.doc
- 《动物免疫学》课程教学资源(文献资料)Introduction to Techniques in Immunology.doc
- 《动物免疫学》课程教学资源(文献资料)Myeloma Proteins.doc
- 《动物免疫学》课程教学资源(文献资料)Natural Killer Cells.doc
- 《动物免疫学》课程教学资源(文献资料)Nonspecific Immunity.doc
- 《动物免疫学》课程教学资源(文献资料)Secondary Lymphoid Organs of the Immune System.doc
- 《动物免疫学》课程教学资源(文献资料)T and B cells 1.doc
- 《动物免疫学》课程教学资源(文献资料)T and B cells 2.doc
- 《动物免疫学》课程教学资源(文献资料)STUDY OF DISEASE AND PHYSIOLOGY IN THE 1979 HOMING STUDY HATCHERY STOCKS——A SUPPLEMENT TO - IMPRINTING SALMON AND STEELHEAD TROUT FOR HOMING, 1979 BY SLATICK, GILBREATH, AND WALCH.pdf
- 《动物免疫学》课程教学资源(文献资料)免疫应答(英文).pdf
- 《动物免疫学》课程教学资源(文献资料)免疫系统的细胞(英文).pdf
- 《动物免疫学》课程教学资源(文献资料)抗体(英文).pdf
