复旦大学:《统计热力学》课程教学资源(课件讲稿)What Theory Can Do(Strength and Weakness)

回H厄与 What Theory Can Do Strength and Weakness
What Theory Can Do Strength and Weakness

Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis mnovative Material 理论计算起到了非常重要的作用 EQUILIBRIUM KINETIC MEASUREMENTS MEASUREMENTS THERMOCHEMICAL KINETICS MECHANISM QUANTUM CHEMICAL CALCULATIONS VALENCY THEORY Fig.1 The linkages between thermochemical kinetics and other subjects Ref.Walsh.R.Chem.Soc.Rev.2008.37.686. 振华制 2013/10/14 What Theory Can Do 造
李 振 华 制 2013/10/14 What Theory Can Do 2 造 理论计算起到了非常重要的作用 Fig. 1 The linkages between thermochemical kinetics and other subjects. Ref. Walsh, R. Chem. Soc. Rev. 2008, 37, 686

Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis mnovative Material Strength For small and regular molecules Chemical Accuracy: Energetics:Error 1 kcal/mol Structures:Error in Bond Lengths <0.01 A Frequencies:A few cm-l 李振华 2013/10/14 What Theory Can Do 3 造
李 振 华 制 2013/10/14 What Theory Can Do 3 造 Strength For small and regular molecules Chemical Accuracy: Energetics: Error < 1 kcal/mol Structures: Error in Bond Lengths < 0.01 Å Frequencies: A few cm-1
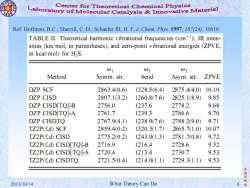
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis&Inovative Material Ref:Hoffman,B.C.;Sherrill,C.D.;Schaefer III,H.F.J.Chem.Phys.1997,107(24),10616 TABLE II.Theoretical harmonic vibrational frequencies (cm),IR inten- sities(km/mol,in parentheses),and zero-point vibrational energies(ZPVE, in kcal/mol)for H2S. 01 02 03 Method Symm.str. bend Asym.str. ZPVE DZP SCF 2863.40.6 1328.5(6.4) 2875.4(4.0) 10.10 DZP CISD 2807.1(3.2) 1260.8(7.6 2825.1(8.9) 9.85 DZP CISD[TQ]-B 2756.0 1237.6 2778.2 9.68 DZP CISD[TQ]-A 2761.7 1239.3 2786.6 9.70 DZP CISDTQ 2767.9(4.1) 1238.9(7.6) 2788.2(9.0) 9.71 TZ2P(f.d)SCF 2859.4(0.2) 1320.3(1.7) 2865.71.0) 10.07 TZ2P(f.d)CISD 2775.2(0.2) 1243.01.3) 2781.7(0.8) 9.72 TZ2P(f,d)CISD[TQ]-B 2716.9 1216.4 2728.6 9.52 TZ2P(f,d)CISD[TQ]-A 2720.4 1213.4 2730.7 9.53 TZ2P(f,d)CISDTQ 2721.5(0.4) 1214.01.1) 2729.31.1) 9.53 振华制 2013/10/14 What Theory Can Do 造
李 振 华 制 2013/10/14 What Theory Can Do 4 造 Ref: Hoffman, B.C.; Sherrill, C. D.; Schaefer III, H. F. J. Chem. Phys. 1997, 107(24), 10616

Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis Innovative Material Ref:Bak K.L.et al.J.Chem.Phys.2001,114(15),6548 TABLE II.Equilibrium bond lengths Re in pm. CCSD(T)/ Molecule Bond Expt. Emp. Emp.-Expt. cc-pCVQZ H2 R 74.144 05 0 74.19 HF Rr 91.680(8 91.69 0.01 9158 H2O RoH 95.72 95.75 0.03 95.71 HOF R OH 96.57(16y 96.78 0.21 96.57 H2O2 RoH 96.71 000 卡0 96.19 HNC RNH 99.40(8 99.49 0.09 99.53 NHy RNN 101.1(6 101.16 0.06 101.12 N2H2 RNH 102.9(1)月 102.86 0.04 102.84 HNO RNH 44 105.17 t年+ 105.24 C2H2 RcH 106.215(17) 106.13 -0.09 106.21 HCN Rc 106.501(8y 106.53 0.03 106.55 C2H RcH 108.1(2y 108.07 0.03 108.09 CHa RcH 108.58(10 108.59 0.01 108.64 N2 RNN 109.768(5 109.77 0.00 109.81 CH2O Rcu 110.0520y 110.07 0.02 110.08 CH2 RcH 110.7(2) 110.63 0.07 110.68 CO Rco 112.832 112.84 0.01 112.89 HCN RcN 115.3242) 115.34 0.02 11538 C02 Rco 115.995m 116.01 0.01 116.04 HNC RcN 116.892) 116.87 -0.02 116.93 C2H2 Rcc 120.257(9P 120.37 0.11 120.37 CH2O Rco 120.33(10y 120.47 0.14 120.43 HNO RNO 年 120.86 g。 120.85 N2H2 RNN 124.71)9 124.57 -0.13 124.67 C2Ha Rcc 133.42y 133.07 -0.33 133.12 F2 RFF 141.193 141.24 0.05 141.13 HOF RFO 143.5031 143.44 -0.06 14326 H2O2 145.56 + 卡, 144.97 振华制 2013/10/14 Roo 5 造
李 振 华 制 2013/10/14 What Theory Can Do 5 造 Ref: Bak K. L. et al. J. Chem. Phys. 2001, 114(15), 6548
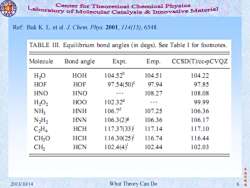
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis Innovative Material Ref:Bak K.L.et al.J.Chem.Phys.2001,114(15),6548 TABLE III.Equilibrium bond angles (in degs).See Table I for footnotes. Molecule Bond angle Expt. Emp. CCSD(Tcc-pCVQZ H2O HOH 104.52 104.51 104.22 HOF HOF 97.54(50)9 97.94 97.85 HNO HNO t 108.27 108.08 H202 HOO 102.32 ,9 99.99 NHy HNH 106.7 107.25 106.36 N2H2 HNN 106.3(2g 106.36 106.17 C2Ha HCH 117.3733) 117.14 117.10 CH2O HCH 116.3025) 116.74 116.44 CH2 HCN 102.4(4 102.44 102.03 李 振华制 2013/10/14 What Theory Can Do 6
李 振 华 制 2013/10/14 What Theory Can Do 6 造 Ref: Bak K. L. et al. J. Chem. Phys. 2001, 114(15), 6548

Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis mnovative Material Ref:Bak K.L.et al.J.Chem.Phys.2001,114(15),6548. Distribution of Equilibrium Bond Length Errors for 19 Small Molecules in a cc- pCVQZ basis-ACES lI results from Bak,et.al.JCP,v.114,p.6548(2001) HF 一MBPT(2) CCSD 一CCSD() -8 -6 -4 -2 0 Error in Bond Lengths (pm) 李振华制 2013/10/14 What Theory Can Do
李 振 华 制 2013/10/14 What Theory Can Do 7 造 Ref: Bak K. L. et al. J. Chem. Phys. 2001, 114(15), 6548
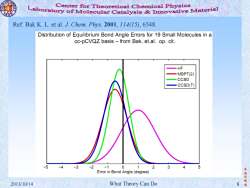
Center for Theoretical Chemical Physics Laboratory of molecular Catalysis Innovative material Ref:Bak K.L.et al.J.Chem.Phys.2001,114(15),6548. Distribution of Equilibrium Bond Angle Errors for 19 Small Molecules in a cc-pCVQZ basis from Bak,et.al.op.cit. -HF MBPT(2) CCSD CCSD(T) -5-4-3-2 -1 0 2 1 3 4 Error in Bond Angle(degree) 振华制 2013/10/14 What Theory Can Do 8
李 振 华 制 2013/10/14 What Theory Can Do 8 造 Ref: Bak K. L. et al. J. Chem. Phys. 2001, 114(15), 6548
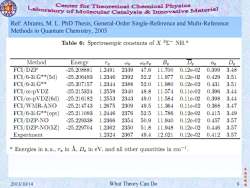
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis Iovative Material Ref:Abrams,M.L.PhD Thesis,General-Order Single-Reference and Multi-Reference Methods in Quantum Chemistry,2005 Table 6:Spectroscopic constants of X 3>-NH. Method Energy Te We WeTe Be De Qe De FCI/DZP -25.208881 1.2491 2339 47.6 11.700 0.12e-02 0.399 3.48 FCI/6-31G*(⑤d) -25.206493 1.2346 2392 52.2 11.977 0.12e-02 0.429 3.51 FCI/6-31G** -25.207157 1.2344 2388 52.0 11.980 0.12e-02 0.431 3.51 FCI/cc-pVDZ -25.215324 1.2559 2340 48.8 11.574 0.11e-02 0.396 3.44 FCI/cc-pVDZ(6d) -25.216182 1.2553 2343 49.0 11.584 0.11e-02 0.398 3.44 FCI/WMR-ANO -25.214743 1.2675 2309 49.5 11.364 0.11e-02 0.388 3.47 FCI/6-31G**(opt) -25.211093 1.2446 2376 52.5 11.786 0.12e-02 0.415 3.49 FCI/DZP-NO -25.229338 1.2366 2354 50.9 11.940 0.12e-02 0.457 3.57 FCI/DZP-NO(5Z) -25.229704 1.2362 2350 51.8 11.948 0.12e-02 0.446 3.57 Experiment 1.2324 2367 49.4 12.021 0.12e-02 0.412 3.57 Energies in a.u.,re in A,De in eV,and all other quantities in cm-1. 振华制 2013/10/14 What Theory Can Do 造
李 振 华 制 2013/10/14 What Theory Can Do 9 造 Ref: Abrams, M. L. PhD Thesis, General-Order Single-Reference and Multi-Reference Methods in Quantum Chemistry, 2005
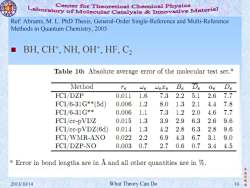
Center for Theoretical Chemical Physics Laboratory of Molecular Catalysis mnovative Material Ref:Abrams,M.L.PhD Thesis,General-Order Single-Reference and Multi-Reference Methods in Quantum Chemistry,2005 BH,CH+,NH,OH+,HF,C2 Table 10:Absolute average error of the molecular test set.a Method Te We WeTe Be De Qe De FCI/DZP 0.011 1.6 7.3 2.2 5.1 2.67.7 FCI/6-31G**(5d) 0.006 1.2 8.0 1.3 2.1 4.47.8 FCL/6-31G** 0.006 1.1 7.3 1.2 2.0 4.6 7.7 FCI/cc-pVDZ 0.015 1.3 3.9 2.9 6.3 2.6 9.6 FCI/cc-pVDZ(6d) 0.014 1.3 4.2 2.8 6.3 2.8 9.6 FCI/WMR-ANO 0.022 2.2 6.9 4.3 6.7 3.1 9.0 FCI/DZP-NO 0.003 0.7 2.7 0.6 0.7 3.4 4.5 a Error in bond lengths are in A and all other quantities are in % 李 振华 2013/10/14 What Theory Can Do 10
李 振 华 制 2013/10/14 What Theory Can Do 10 造 Ref: Abrams, M. L. PhD Thesis, General-Order Single-Reference and Multi-Reference Methods in Quantum Chemistry, 2005 BH, CH+ , NH, OH+ , HF, C2
按次数下载不扣除下载券;
注册用户24小时内重复下载只扣除一次;
顺序:VIP每日次数-->可用次数-->下载券;
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)06 统计热力学的应用——反应速率的统计理论.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)06 统计热力学的应用——理想气体的平衡常数.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)06 统计热力学的应用——振动配分函数的计算.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)06 统计热力学的应用——多原子分子转动,内转动.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)06 统计热力学的应用——气体(理想气体,真实气体).pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)05 配分函数和热力学函数.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)04 Chapter 4 经典统计和量子统计.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)03 系综理论(近独立或自由粒子系统).pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)02 Mathematics.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)Introduction.pdf
- 《统计热力学》课程教学资源(参考文献)三体问题(Three Body Problem).doc
- 《统计热力学》课程教学资源(参考文献)From 奇迹笔记——从落体到无线电波经典物理学家和他们的发现.doc
- 《统计热力学》课程教学资源(参考文献)吉布斯(1839~1903)Gibbs,Josiah Willard.doc
- 《统计热力学》课程教学资源(参考文献)玻耳兹曼(1844-1906).doc
- 复旦大学:《数理统计在化学中的应用》课程教学资源(课件讲稿)07 机器学习基础.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)06 统计热力学的应用.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)第六章 测量误差与测量不确定度.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)数理统计方法在化学中的应用(随机变量和分布函数、正态分布).pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)01 Chemical Statistical Thermodynamics(Introduction,主讲:李振华).pdf
- 《统计热力学》课程教学资源(课件讲稿)Insight into entropy.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)07 Monte Carlo方法及其在化学中的应用 §7.1 Monte Carlo模拟.pdf
- 复旦大学:《统计热力学》课程教学资源(课件讲稿)07 Monte Carlo方法及其在化学中的应用 §7.2 分子动力学模拟 模拟分子动力学模拟及其在化学中的应用.pdf
- 《统计热力学》课程教学资源(参考文献)Allen. M.P.Introduction to MD.pdf
- 《统计热力学》课程教学资源(参考书籍)Computational molecular dynamics - challenges, methods, ideas.pdf
- 《统计热力学》课程教学资源(参考书籍)B·J·麦克莱兰:统计热力学(1980,共十四章).pdf
- 《统计热力学》课程教学资源(参考文献)GodehardSutmann:Classical Molecular Dynamics.pdf
- 《统计热力学》课程教学资源(参考文献)Levine R. D.:Introduction to reactive molecular collisions.pdf
- 《统计热力学》课程教学资源(参考书籍)李政道:统计力学(1984,共四章).pdf
- 普通高等教育“十五”国家级规划教材:《统计物理学》PDF电子书(第二版,编著:苏汝铿,共十章).pdf
- 《统计热力学》课程教学资源(参考书籍)《统计热力学导论》PDF电子书(编著:赵成大,1983,共十二章).pdf
- 《统计热力学》课程教学资源(参考书籍)Allen MP & Tildesley DJ:Computer Simulation of Liquids.pdf
- 科学出版社:《分子热力学》PDF电子书(1990,共十章,编著:金家骏、陈民生、俞闻枫).pdf
- 化学工业出版社:《分子模拟——从算法到应用》PDF电子书【荷】Frenkel & Smit(2002,共五部分)UNDERSTANDING MOLECULAR SIMULATION - FROM ALGORITHMS TO APPLICATIONS.pdf
- 《量子化学基本原理和从头计算法》PDF电子书(编著:徐光宪,上、中、下册,共二十四章,1989年第一版,各章含习题).pdf
- 复旦大学:《统计热力学》课程教学资源(作业习题)练习一(含答案).doc
- 复旦大学:《统计热力学》课程教学资源(作业习题)练习三.doc
- 复旦大学:《统计热力学》课程教学资源(作业习题)练习三答案.doc
- 复旦大学:《统计热力学》课程教学资源(作业习题)练习二 答案.doc
- 复旦大学:《统计热力学》课程教学资源(作业习题)练习二.doc
- 复旦大学:《统计热力学》课程教学资源(作业习题)练习五.doc
